In recent years, the resilience and security concerns of the US drug supply chain have grown rapidly.
It prompted a closer examination of the vulnerabilities of the US Drug Supply Chain and the role of federal agencies in ensuring its integrity.
A new report sheds light on the current state of the US drug supply chain, emphasizing the need for enhanced accountability measures.
The Current State of the US Drug Supply Chain
The US drug supply chain is a complex network of
- Manufacturers
- Wholesalers
- Distributors
- Pharmacies
- Healthcare providers
While it generally operates effectively, recent incidents have revealed vulnerabilities that pose significant risks.
According to a new report from the Senate Committee on Homeland Security and Governmental Affairs, the Lack of transparency in the US drug supply chain has limited the government’s ability to predict and address the nation’s rising number of critical drug shortages.
The committee noted that the shortages peaked at 295 at the end of 2022, including both prescription and over-the-counter drugs.
The drugs are used in a range of areas from emergency departments in hospitals to relieve cold and flu symptoms in children.
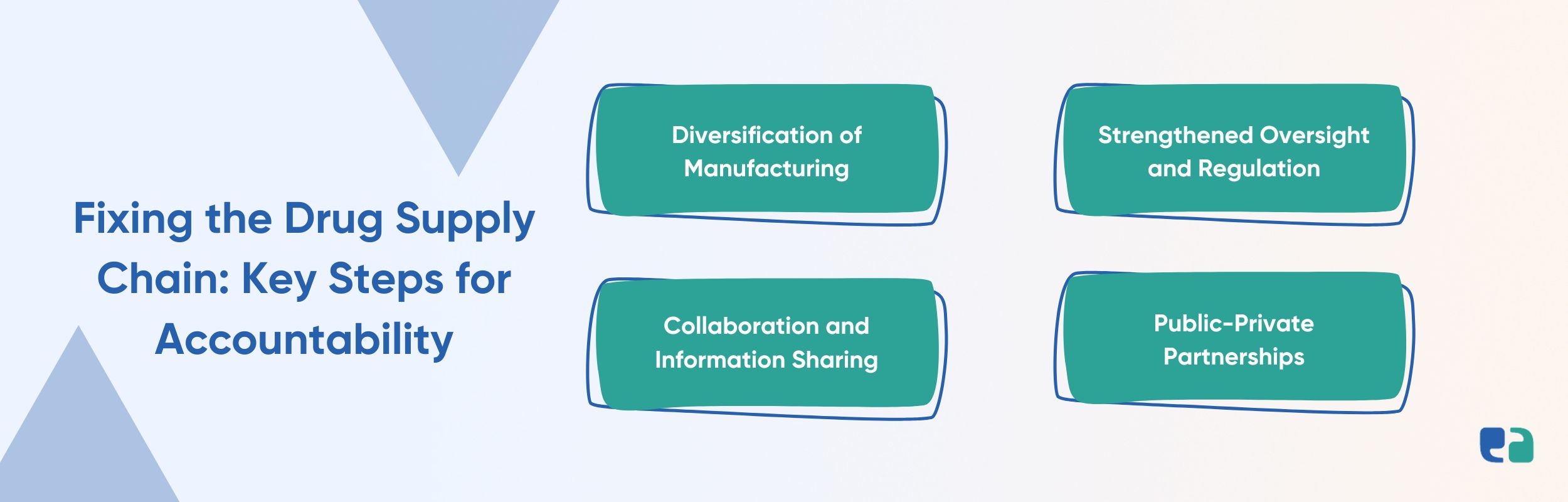
Ways To Enhance Accountability in the US Drug Supply Chain
To address the issues identified in the report and ensure a resilient drug supply chain, federal agencies must take concrete steps to enhance accountability. Here are some key recommendations:
Achieve the Goal of a Resilient and Secure Drug Supply Chain with Us!
Public-private partnerships play a crucial role in strengthening the supply chain.
SyS Creations is a healthcare software development company and can contribute its expertise and innovative solutions to this endeavor.
By implementing these measures, the US drug supply chain can become more
- Secure
- Transparent
- Responsive
Ultimately, protecting the availability and safety of essential medications.
We, at SyS Creations, aim to strengthen the entire supply chain.
Together, we can bolster the integrity of the pharmaceutical industry and ensure the continuous availability of vital medications for the well-being of the population.



